Hematopoietic stem cell transplantation is a well-established efficient therapy for hematological diseases, but Graft-Versus-Host Disease (GVHD) is a major and frequent complication encumbering its outcome despite the administration of calcineurin inhibitor based GvHD-prophylaxis. Corticosteroids represent the worldwide first line treatment, however in case of steroid refractory acute GVHD there is no consensus about a subsequent treatment although Ruxolitinib is subject to a phase III trial. Multiple molecules have been tried, most of them immunosuppressive, increasing the risk of deadly infections and transplantation-related mortality (TRM). Recent studies reported that mesenchymal stromal cells (MSC) infusion which have immune modulatory abilities might be effective and harmless in steroid or treatment-refractory GVHD (R-GVHD). In France, MSCs are considered as an Advanced Therapy Medicinal Product. For 4 years, its administration as a compassionate use is subject to approval by an expert committee from SFGM-TC before its validation by the French regulatory agency (ANSM).
We retrospectively analyzed the demands for MSC use in France since 2011 for patients suffering from R-GVHD.
We evaluated the response at day 28 (range 23-28) and at the last follow-up and its safety.
Eleven demands were validated by both expert committee and ANSM, 8 patients (pts) received ex-vivo expanded MSCs, 1 pt refused the therapy, 1 infusion was postponed due to COVID-19 related sanitary crisis and the last 1 didn't receive MSCs due to relapse.
Among pts who received MSCs, median age was 6 years (2-69), sex ratio was 0,6.
All pts underwent their first HSCT for either malignant disease (62,5%) or non-malignant disease (37,5%). Four pts were transplanted from sibling donor, 2 pts from mismatched unrelated donors and 2 pts from haplo-identical donors. Stem cells source was bone marrow for 4 pts, peripheral blood stem cells for 3 and cord blood for 1. Donors median age was 28,8 years (0-49,5), 1 male had a female donor. Six pts got a myeloablative conditioning regimen (TBI-based for 2). All pts received a ciclosporin-based (CSA) GVHD prophylaxis (CSA alone, n=1; CSA + Mycophenolate Mofetil (MMF) or Methotrexate, n=7). Five pts had ATG.
Six pts were suffering from acute GVHD, while 2 from extensive chronic GVHD (cGVHD). All 6 pts with acute GVHD presented a grade III or IV, refractory to corticosteroids and at least 2 other lines of GVHD therapy. All but one had a multipolar GVHD with at least 2 affected organs. Five pts were still taking corticosteroids, and six were taking additional immunosuppressive molecules (Tacrolimus, Ruxolitinib, Etanercetp, Inolimomab, MMF) at time of MSC infusion.
Five pts received German commercialized MSCs (Obnitixâ, MEDAC, Germany; see Bader et al, 2018), 2 get mother's derived MSCs (not the initial donor), and 1 from a third-party donor.
A median of 4 infusions were administered (1-4), once a week for 4 weeks. Mean single dose of MSCs was 1.23.10e6/kg (range: 0,86 - 3). No toxicity was reported except for 1 pt who experienced anaphylactic reaction within minutes, leading to the interruption of infusion (mother's derived MSCs prepared with fetal bovine serum where all other preparations were performed with platelet lysate). The median time from GVHD onset to first MSC infusion was 135 days (63-457). Overall response rate was 86% (6/7) at the first and at the last evaluation with 1 complete response (CR) and 5 partial responses (reduction of at least one grade of at least one affected organ). One pt did not respond and the last 1 was not evaluable due to anaphylactic reaction. Both were suffering from cGVHD.
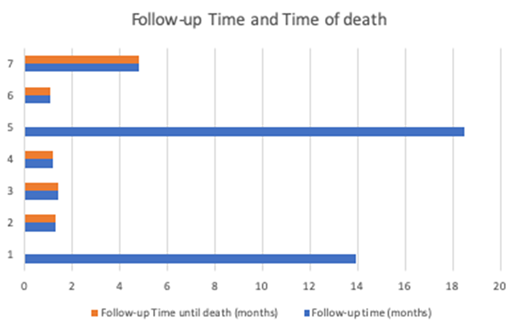
Among the seven pts who received complete MSC infusions, median follow-up was 1,5 months (1,1-18,5) due to premature TRM, overall survival (OS) at six months was 33,3%.
Five pts died, all of them from a transplantation-related cause: GVHD n=2, severe infections n=3.
Literature reported better outcomes lately, Bader and al, 2019 reported a 64% OS at 6 months and 51% of CR at last follow up. Those disparities might be explained by a delayed treatment after GVHD onset (135 days versus 28 days) and a median of 3 (2-10) therapies after receiving corticosteroids before MSC infusion due to difficulty to obtain MSCs in France. Besides, we included patient suffering from R-cGVHD.
Regarding those results, MSC efficacy and safety should be confirmed in a proper clinical trial.
Rubio:Neovii: Research Funding; Novartis: Honoraria; MSD: Honoraria; Gilead: Honoraria; Medac: Consultancy. Dalle:Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; AbbVie Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bellicum: Consultancy, Honoraria; Medac: Consultancy, Honoraria; Orchard: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal